Egnyte for Life Sciences
Simplify Statistical Data Analysis
A centralized statistical computing environment for research and development that integrates all of your data, metadata, programs, and results - streamlining your statistical analyses
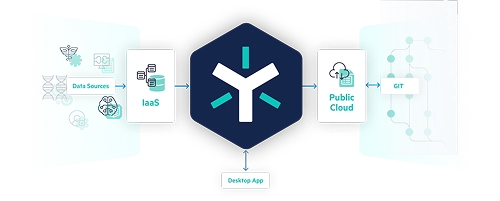
Common Challenges in a Statistical Computing Environment
Workflow
- Integrity issues observed during consolidation of clinical data
- Insufficient automation and workflows for reviewing and approving data
- Lack of defined configuration to ensure adherence to business process rules
Governance
- Tedious manual procedures to establish data integrity for regulatory submissions
- Unavailability of a readily deployable validated framework
- Restricted capability to consolidate audit trails
Open Access
- Insufficient support for GXP and non-GXP exploratory studies
- Lack of open API for seamless integration
- Inadequate support for programming languages such as SAS, R, and Python
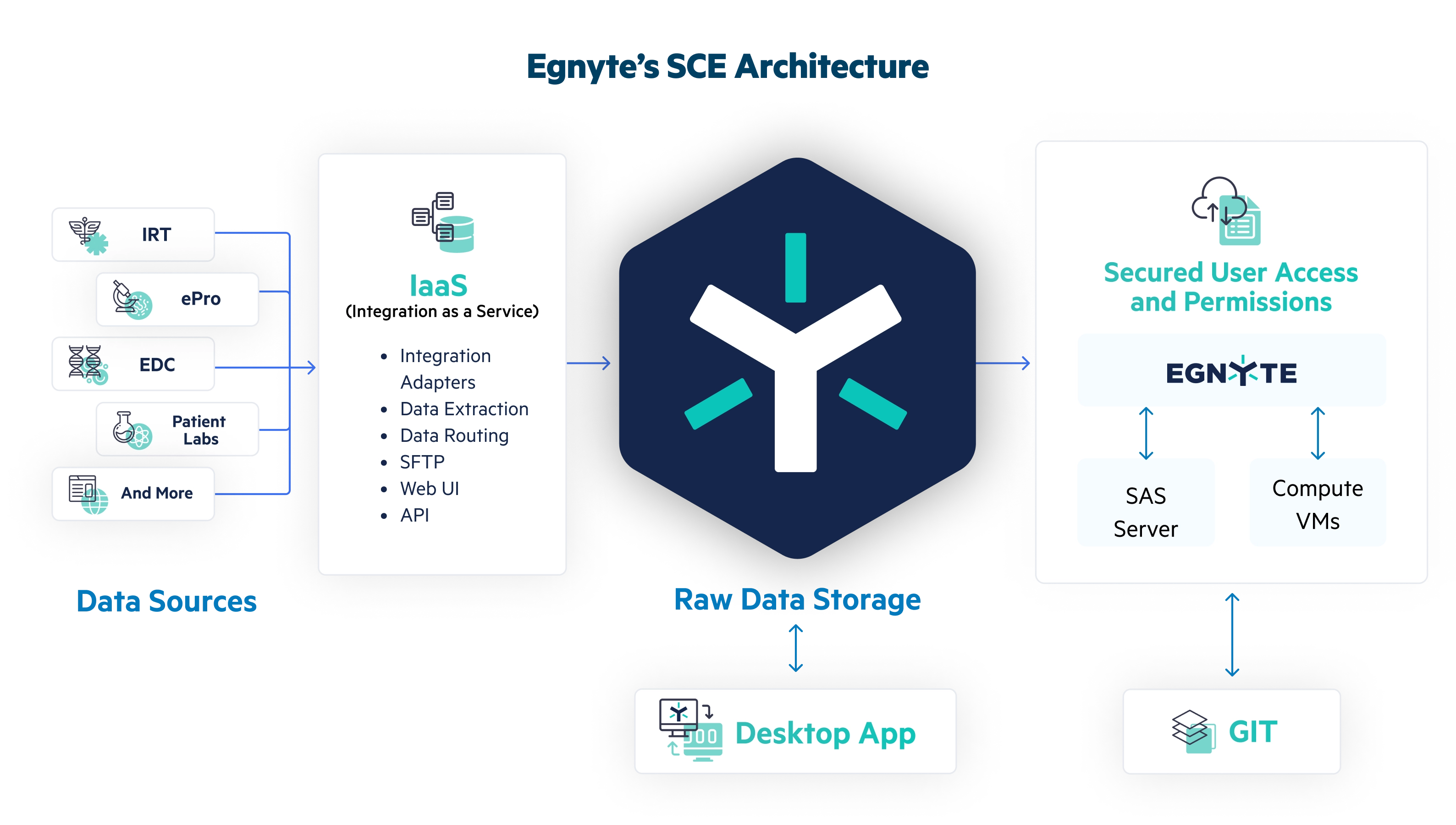
The Egnyte Solution
Operational Efficiency
- Deploy seamlessly for local, hybrid, and cloud for real time access to data
- Mitigate risks with Egnyte’s built-in granular security feature
- Ensure compliance with simplified access to audit trails
Centralized Data Repository
- Aggregate clinical data from diverse sources via SFTP, Web UI or APIs
- Version control available for files in a GxP validated environment
- Multi-lingual support for SAS, R, and Python applications
Secure Collaboration
- Permission users to effortlessly share files, internally and across your partner ecosystem
- User-defined workflows for document review and approval
- Fine-grained governance controls and reporting for various types of users and content
Egnyte Works the Way You Do

Making life easier for Scientists and Lab Personnel by Connecting to the Data and Tools that Matter Most
Transforming Statistical Analysis
The Power of Many Tools in One Turnkey Platform
Egnyte’s modular offerings grow with your company through all development and approval phases - from startup to scale up.
Egnyte
- Secure Collaboration with Partners
- Workflow Orchestration
- Authorized Access Controls
- Data Management
- Sensitive Data Discovery
- Document Lifecycle Management
Egnyte for Life Sciences
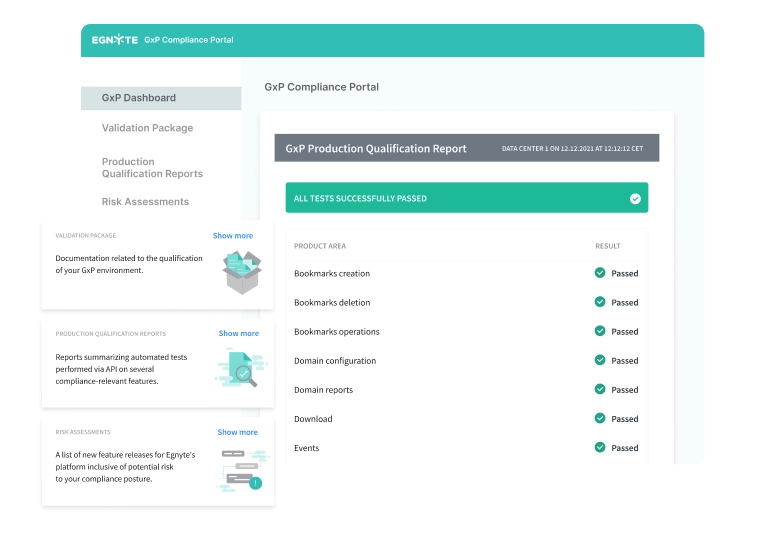
- GxP Compliance Portal
- Validation as a Service
- Life Science Specific Integrations
- eTMF Management and Archival
- Controlled Documentation and Training


