Strengthen Data Governance in Life Sciences
Secure sensitive data, ensure audit and inspection readiness, and prepare your data for AI.

Modernize Governance Across Research,
Clinical, and Regulatory Data
Identify and protect sensitive content, enforce data standards, and establish
complete control with Egnyte’s unified data governance platform.
complete control with Egnyte’s unified data governance platform.
Automate Oversight and Reduce Risk
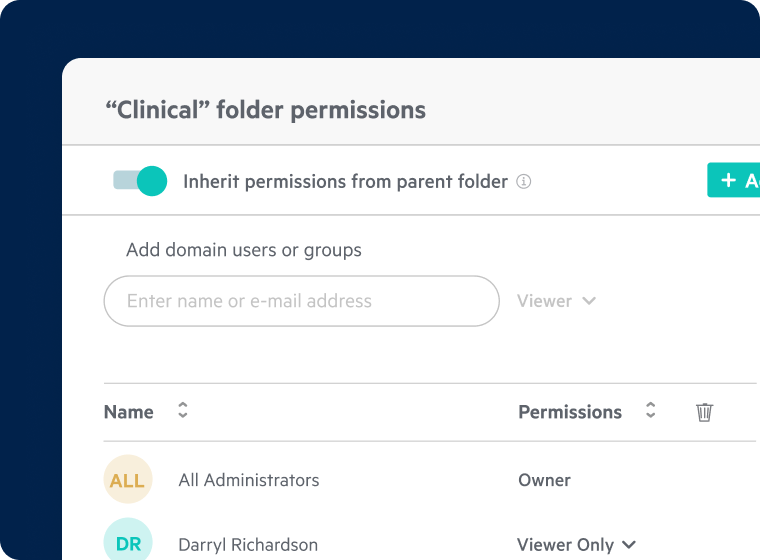
Granular Access and Sharing Controls
- Enable role-based permissions across internal and external users
- Offer secure, compliant collaboration with CROs, sites, and partners
- Use secure enclaves for statistical and regulated data
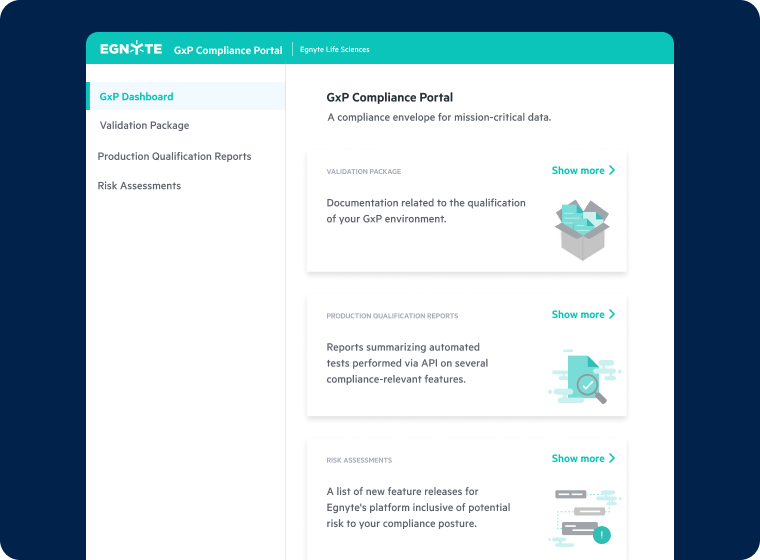
Unified Audit Trails and Compliance Monitoring
- Centralize audit logs and track activity for regulated files
- Implement built-in controls for FDA, EMA, ALCOA+, and ICH E6 (R3) compliance
- Achieve readiness for audits and inspections with real-time visibility
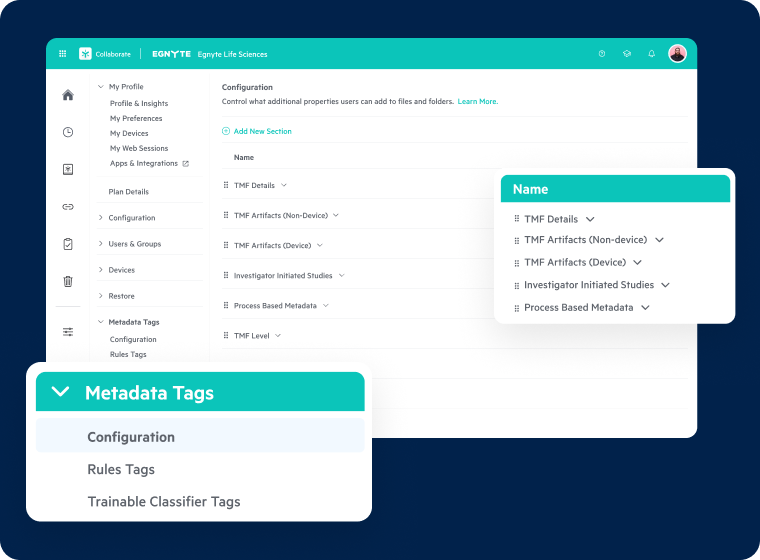
AI-Powered Governance and Metadata Management
- Ensure consistency for SOPs and quality docs with AI-driven tagging
- Enhance search capabilities across regulated and research data
- Preserve data integrity and streamline validation documentation
Integration With Leading
Life Sciences Applications
Egnyte integrates seamlessly with your systems, making it easy to manage, collaborate on, and maintain compliance for your clinical files.






